Introduction
In modern medicine—particularly in invasive and specialized surgeries—infection control and patient safety are fundamental principles directly linked to surgical success and the reduction of postoperative complications. The transmission of microbes, bacteria, or contaminated particles within the operating room can lead to hospital-acquired infections (HAIs), which not only threaten patient health but also significantly increase treatment costs and recovery time.
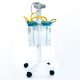
To prevent such risks, the use of sterile disposable medical devices has become a highly effective preventive strategy. One of these essential devices is the sterile disposable suction bag, specifically designed to collect and manage fluids, blood, secretions, and even extracted tissues during surgical procedures. These bags, made from medical-grade materials, feature a durable mechanical design and sterile packaging to ensure maximum safety for both patients and medical staff. They can be used directly in the operating room without the need for any additional disinfection process.
Sterile disposable suction bags are particularly important in aesthetic and liposuction surgeries, where the collected fat or tissue may be re-injected into other areas of the body. In such cases, a fully sterile and reliable suction bag not only ensures patient safety but also preserves the purity and quality of the collected tissue.
Given the growing importance of international standards in medical device manufacturing, these bags must comply with ISO 10993, ISO 13485, CE, and FDA requirements to guarantee their safety and performance. The use of sterile disposable suction bags, now considered a global standard in operating rooms, exemplifies the integration of material science, biomedical engineering, and clinical hygiene—all designed with the singular goal of protecting patients’ health and lives.
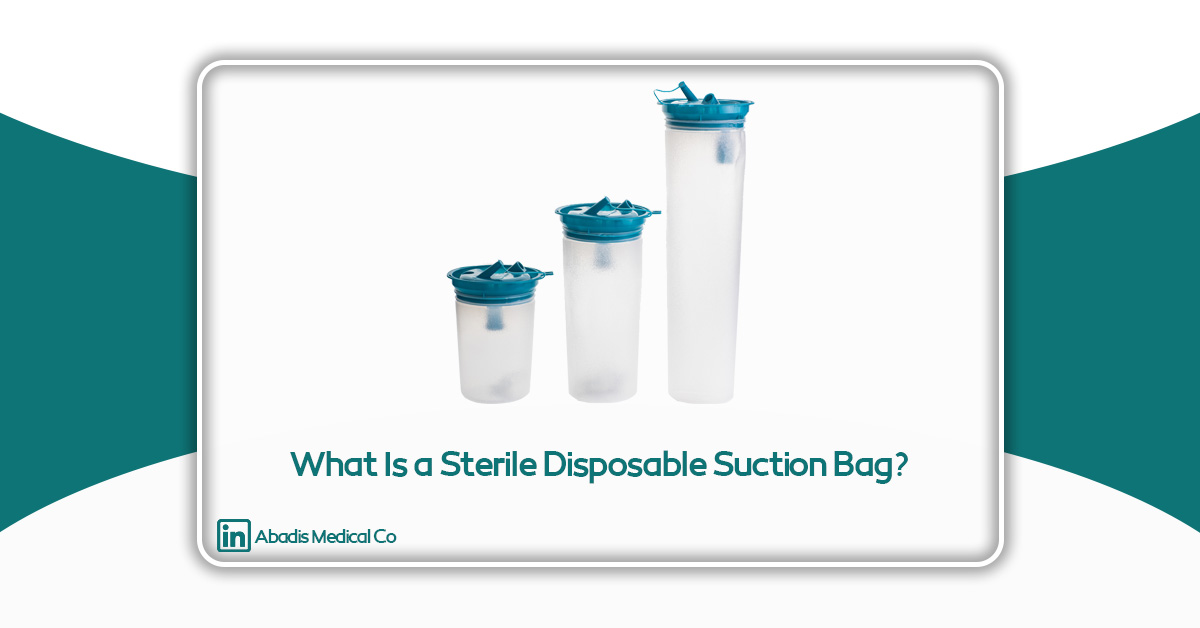
Definition: What Is a Sterile Disposable Suction Bag?
A sterile disposable suction bag is a flexible yet durable container that connects to suction devices to collect secretions, blood, and bodily fluids. Its key characteristics are sterility and single-use design—meaning the bags are produced and sealed under controlled sterile conditions and can be used directly in sensitive medical environments such as operating rooms, without any prior washing or sterilization.
Applications of Sterile Disposable Suction Bags
Because of their technical, hygienic, and sterile properties, sterile disposable suction bags are widely used in various medical and surgical settings. Their use enables the safe and contamination-free collection of fluids, blood, secretions, and extracted tissues. Below are the main applications:
1. Plastic and Aesthetic Surgeries (Liposuction)
-
Collection of extracted fat during liposuction and fat transfer procedures while maintaining purity and tissue integrity.
-
Reduction of infection risks caused by contact with biological fluids or contaminated materials.
-
Allowing direct re-injection of contents into the body under sterile conditions.
-
Transparent bag design enables real-time visual monitoring of the collected fat volume.
2. General and Orthopedic Surgeries
-
Collection of blood, fluids, and secretions during tumor removal or joint surgery.
-
Reduction of excess fluids in the surgical field for improved visibility.
-
Prevention of contamination in the operating room and minimizing exposure risks for medical staff.
3. Cardiovascular Surgeries
-
Use during long cardiac or vascular operations to collect blood and excess fluids.
-
Protection of suction systems from backflow contamination.
-
Reduced risk of microbial transmission in highly sensitive cardiac environments.
4. Emergency Rooms and Intensive Care Units (ICU)
-
Rapid and safe fluid and secretion collection in critical and emergency situations.
-
Ready-to-use design eliminates the need for preparation or cleaning.
-
Reduces contamination risk and safeguards healthcare workers in high-pressure settings.
5. Dental and ENT Clinics
-
Collection of saliva, blood, and respiratory secretions.
-
Prevention of microbial contamination and improved patient and staff safety.
-
Simplified management of fluids during short and specialized procedures.
6. Specialized Surgeries Requiring Sample Storage
-
Suitable for procedures where collected fluids or tissues are later analyzed in laboratories.
-
Sterile bags ensure that samples remain uncontaminated and preserve their integrity for testing.
7. General Advantages Across All Fields
-
Eliminates the need for cleaning and re-sterilization—saving time and operational costs.
-
Reduces the risk of hospital-acquired infections (HAIs) for both patients and medical staff.
-
Complies with global ISO and FDA safety and hygiene standards.
-
Enhances surgical efficiency and operating room management during complex procedures.
Difference Between Sterile Suction Bags and Regular Suction Bags
|
Feature |
Regular Suction Bag | Sterile Single-Use Suction Bag |
| Hygienic Condition | Usually non-sterile or semi-sterile; suitable for general environments |
Fully sterile, free from microbial contamination; ideal for critical and sensitive surgeries |
|
Main Application |
General wards, emergency units, and short-term care |
Operating rooms and long, complex surgical procedures (e.g., liposuction) |
|
Content Storage Capability |
Mostly designed for immediate disposal |
Allows temporary storage of extracted fat or bodily fluids |
|
Reusability |
Single-use, no need for washing or disinfection |
Single-use, no need for washing or disinfection |
| Standards and Compliance | Limited regulatory requirements |
Strict compliance with international standards (ISO, CE, FDA) |
Special Importance in Liposuction Surgery
Liposuction is one of the most popular cosmetic surgeries in the world, during which excess body fat is suctioned and removed. In this process, the selection of an appropriate suction bag is of great importance:
-
Preservation of Extracted Fat Quality: The extracted fat is often reinjected into other areas of the body, and only sterile bags can ensure its safe storage.
-
Infection Prevention: Using non-sterile bags increases the risk of contamination and infection.
-
Safety of the Surgical Team: Sterile bags are equipped with safety filters that prevent contamination transfer.
-
Global Standard: According to international surgical protocols, the use of sterile suction bags in liposuction is mandatory.
Technical Features of Sterile Single-Use Suction Bags
-
Medical-grade raw materials (PVC or PE)
-
High transparency
-
Mechanical resistance against tearing and leakage
-
Flow shut-off filter
-
Various capacities (1 to 3 liters)
-
Sterile, single-use packaging
Standards and Certifications
-
ISO 13485: Quality management system for medical devices
-
ISO 10993: Biological evaluation and biocompatibility of materials
-
USP Class VI: Safety standard for polymeric materials
-
CE Marking: European Union safety compliance
-
FDA 21 CFR Part 820: U.S. FDA medical device regulations
Advantages of Using Sterile Single-Use Suction Bags
-
Reduced risk of hospital-acquired infections (HAIs)
-
Improved surgical outcomes and shorter recovery times
-
Enhanced safety for medical staff
-
Time and cost efficiency
-
Compliance with global health and safety protocols
-
Safe use in long and complex surgical procedures
Conclusion
The sterile single-use suction bag is one of the essential devices in modern operating rooms, playing a crucial role in enhancing patient safety, reducing infection risk, and improving the quality of surgical outcomes. Its use, particularly in complex and lengthy surgeries such as liposuction, cardiovascular, and orthopedic procedures, ensures that fluids, blood, and tissues are managed with maximum safety and precision.
The key differences between sterile and regular suction bags include complete sterility, medical-grade materials, anti-backflow systems, high transparency, and superior mechanical durability. These features allow for direct use in the operating room without the need for re-sterilization and minimize exposure to contaminated fluids.
From a regulatory perspective, sterile suction bags comply with ISO 10993 (biocompatibility), ISO 13485 (medical device quality management), CE Marking, and FDA 21 CFR Part 820 standards, ensuring patient safety and alignment with international medical protocols.
Clinically, these bags are used not only in cosmetic and liposuction surgeries but also in general surgery, orthopedics, ICU, emergency departments, dental, and ENT clinics. Their clinical benefits include reduced infection risks, preserved sample quality, easy handling, and rapid setup.
Economically, sterile suction bags reduce the costs of re-sterilization, save operating room preparation time, and increase healthcare team productivity. Furthermore, by lowering the risk of hospital-acquired infections, they help reduce postoperative treatment costs while improving patient safety.
Ultimately, the sterile single-use suction bag represents the integration of material science, biomedical engineering, and clinical hygiene. With emerging innovations such as smart, biodegradable, and high-pressure-resistant suction bags, the future of this field points toward continuous improvement in safety, efficiency, and healthcare quality.
Therefore, the use of sterile single-use suction bags is not only a modern surgical standard but also a strategic tool for improving patient safety and optimizing surgical performance — solidifying its position as the gold standard in fluid and tissue management.
References
-
RAUMEDIC, “The Important Role of PVC in Medical Devices and Applications.”
-
European Council of Vinyl Manufacturers (ECVM), “PVC in Healthcare.”
-
Kent Elastomer, “Medical-Grade PVC Tubing: Benefits and Uses.”
-
ISO 10993, “Biological Evaluation of Medical Devices – Part 1: Evaluation and Testing within a Risk Management Process.”
-
ISO 13485, “Medical Devices — Quality Management Systems — Requirements for Regulatory Purposes.”
-
FDA 21 CFR Part 820, “Quality System Regulation for Medical Devices.”