In healthcare facilities, infection control is one of the fundamental criteria for maintaining the health of patients and medical staff. Disposable suction bag systems, due to direct contact with bodily fluids, blood, and contaminated particles, are among the most sensitive points in the transmission of hospital-acquired infections. Any defect in filtration can facilitate contamination of the central suction pump, suction tubing, hospital environment, and even healthcare personnel.
Abadis Company has developed a series of viral-antibacterial filters and sintered safety flow-stopper filters, providing a scientific, reliable, and fully internationally standardized solution to ensure safety in suction systems.
The Importance of BFE and VFE Indicators in Medical Filtration
The performance of hospital filters is usually measured by two key indicators: BFE and VFE. These indicators determine the filter’s ability to block pathogens.
BFE (Bacterial Filtration Efficiency)
BFE represents the efficiency of bacterial filtration, indicating the percentage of bacteria that a filter can remove.
-
For example, a BFE > 99.999% means the filter stops nearly all bacteria.
-
In environments such as operating rooms, ICU, and CCU, this performance is crucial to prevent contamination.
VFE (Viral Filtration Efficiency)
VFE measures the filter’s efficiency in blocking viral particles. Since viruses are smaller than bacteria, achieving:
-
VFE > 99.999% indicates a highly reliable performance in removing viruses.
Together, these indicators define the efficiency of viral-antibacterial filters.
Global Standards for BFE and VFE Testing
Professional suction filters must be tested according to recognized international standards, including:
-
ASTM F2101: The main standard for measuring bacterial filtration efficiency (BFE).
-
EN 14683: European standard for medical masks and filters, applicable for testing filter performance.
Laboratory Methods for VFE
-
Use of standardized viruses under controlled conditions to determine viral filtration efficiency.
Compliance with these standards ensures the filter maintains reliable performance in real clinical conditions.
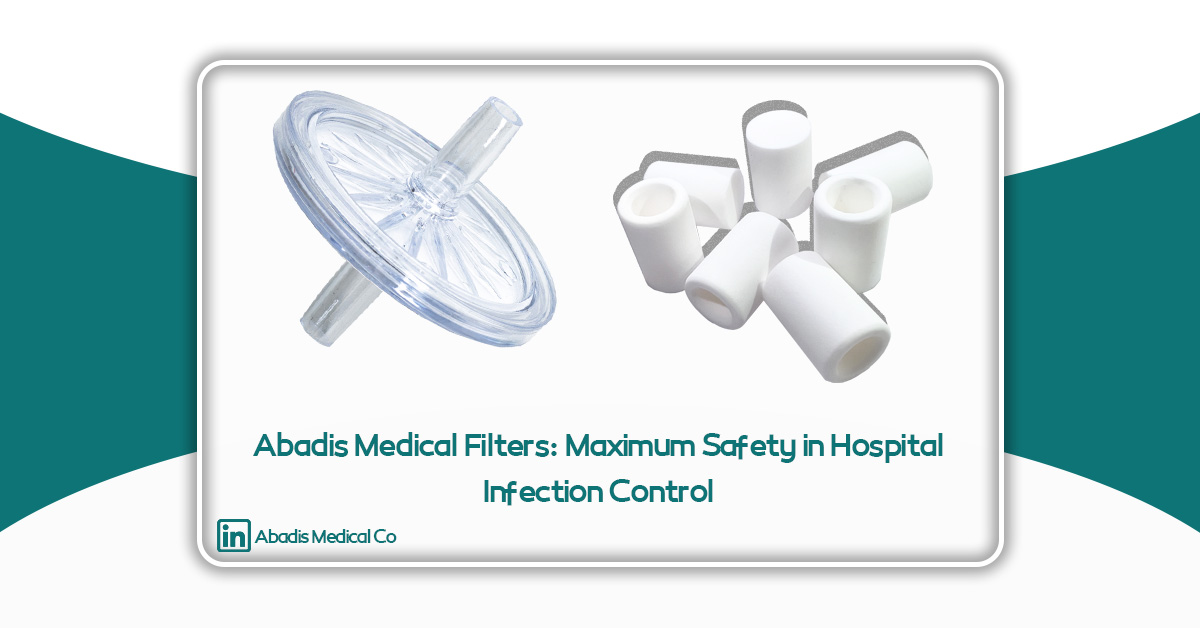
Product 1: Abadis Antibacterial Filter (Viral & Antibacterial Filter)
VFE/BFE > 99.999% | Simultaneous Virus and Bacteria Filtration Efficiency
The Abadis Antibacterial Filter, also known in the industry as the Viral Filter, is a multi-layer filter with extremely high efficiency in removing viral and bacterial particles. It is designed for installation on disposable suction bags and prevents aerosols and contaminated particles from entering the central suction system.
Advantages:
-
Extremely high efficiency in removing viruses and bacteria
-
Reduces the risk of hospital-acquired infections
-
Prevents contamination of the pump and central tubing
-
Protects healthcare personnel during bag handling
-
Stable performance even under high suction volumes
Applications:
-
Operating Rooms
-
ICU and CCU
-
Emergency Departments
-
Infectious and Isolation Wards
-
Pediatric and Neonatal Intensive Care Units
Product 2: Abadis Sintered Flow-Stop Filter (Sintered Safety Filter)
Complete Protection for Central Suction System | Automatic Flow Cut-off in Case of Overflow
The Abadis Sintered Filter, also called the Overflow Protection Filter, provides a critical safety layer in suction systems. When the suction bag fills with fluid, this filter automatically blocks flow entirely, preventing effluents from entering the central pump.
Advantages:
-
Complete protection of the central suction system
-
Prevents pump damage and system contamination
-
Automatic shut-off in case of bag fill or overflow
-
Durable and dense structure using Sintered technology
-
Compatible with Abadis disposable suction bags
Importance:
The suction system is a vital component in operating rooms. Entry of fluids into the central pump can cause:
-
Impeller damage
-
Contamination of the pathway
-
System shutdown
-
Potential risks for patients
The Sintered Flow-Stop Filter prevents all these issues.
Why Abadis Filters Are Essential for Suction Systems
-
Reduces the risk of hospital-acquired infections
-
Provides complete protection for the central suction system
-
Ensures safety for both healthcare personnel and patients
-
High quality and durability in line with international standards
-
Compatible with all Abadis suction bag models
-
Reliable performance in high-risk environments
Using these filters significantly increases the safety level of healthcare facilities and prevents costly consequences.
Summary
Abadis Filters — including the Viral & Antibacterial Filter and the Sintered Safety Flow-Stop Filter — provide a professional and standardized solution for infection control and protection of hospital suction systems. With BFE/VFE > 99.999%, effective removal of contaminated particles, and central pump protection, these filters play a key role in creating a safe, clean, and reliable clinical environment.
Together with Abadis disposable suction bags, these two complementary products form a complete infection control system for hospitals that is globally standardized, sustainable, and fully reliable.
References
-
ASTM F2101-19: Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials. ASTM International.
-
EN 14683:2019 – Medical face masks — Requirements and test methods. European Committee for Standardization (CEN).
-
Rengasamy, S., Eimer, B., & Shaffer, R. (2009). Evaluation of the Filtration Performance of Various Respiratory Masks. Annals of Occupational Hygiene.
-
Oberg, T., & Brosseau, L. (2008). Surgical mask filter and fit performance. American Journal of Infection Control.
-
Hinds, W. C. — Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Wiley-Interscience.
-
CDC — Centers for Disease Control and Prevention. Guidelines for Preventing Transmission of Infectious Agents in Healthcare Settings.
-
WHO — World Health Organization. Infection Prevention and Control in Health Care Facilities.
-
Nelson Labs White Papers. Standards for viral filtration efficiency testing using bacteriophage phiX174 and aerosolized viral models.