Scientific, Operational, and Managerial Guide Based on WHO and CDC Standards
Introduction
The rise of microbial resistance, the spread of infectious diseases, and ever-increasing patient-safety requirements have compelled healthcare facilities to strictly enforce infection-control standards at all levels of operation. One often overlooked source of contamination is suction devices, which are used to collect secretions, blood, fluids, and infectious materials from the patient. Direct contact of these materials with equipment surfaces, staff, and the surrounding environment—if not properly managed—can lead to healthcare-associated infections (HAIs), including hepatitis, HIV, MRSA, and other pathogens.
Suction systems are typically configured in two ways:
- Reusable suction canisters
- Disposable suction liners
This guide, founded on authoritative sources from the World Health Organization (WHO), the U.S. Centers for Disease Control and Prevention (CDC), and the Association for the Advancement of Medical Instrumentation (AAMI), provides a detailed evaluation of cleaning methods, advantages, drawbacks, and best-practice recommendations for both systems.
Section One: Cleaning and Disinfection of Reusable Suction Canisters
1.1 Structural Characteristics
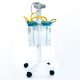
Reusable suction canisters are generally constructed from polycarbonate or polypropylene, offering resistance to heat and chemicals. These canisters are integrated into a hospital’s central suction infrastructure and must be cleaned, disinfected, and dried scientifically after each use.
1.2 Standard Cleaning Protocol (According to WHO & CDC)
Step 1: Preventive Measures & Personal Safety
- Use full PPE: heavy-duty gloves, N95 or surgical mask, safety goggles, and waterproof gown
- Ensure proper ventilation in cleaning area
- Wash hands before and after the procedure
Step 2: Contents Disposal
- Empty fluids into biohazard containers or approved hospital wastewater systems
- For highly infectious secretions (e.g., respiratory pathogens), use dedicated labeled containers
Step 3: Physical Cleaning
- Scrub the interior using dedicated brushes
- Clean with warm water (45–60 °C) and mild or enzymatic detergent
Step 4: High-Level Disinfection
- Use one of the following:
- 0.5% sodium hypochlorite for at least 10 minutes
- 2% glutaraldehyde or peracetic acid as per manufacturer’s guidelines
- Monitor contact time and ensure adequate ventilation
Step 5: Rinsing with Sterile Water
- Eliminate chemical residues completely
- Optional: autoclave using appropriate temperature/pressure cycles
Step 6: Drying & Storage
- Air dry or wipe with lint-free sterile cloths
- Store in enclosed, dust-free cabinets
- Record cleaning date, time, and responsible staff on tracking forms
1.3 Safety Considerations & Common Mistakes
- High-concentration hypochlorite can corrode canisters
- Inadequate drying may foster mold or bacterial growth
- Discontinue use of canisters showing cracks or significant discoloration
Section Two: Disposable Suction Liners – Design, Benefits, and Safe Handling
2.1 Functional Design
Disposable liners consist of medical-grade multilayer plastic bags equipped with secure lids. They are placed inside reusable canisters and collected post-procedure without opening or direct contact.
2.2 Advantages
- Eliminates disinfection requirements and associated infection risks
- Enables rapid replacement, ideal for emergencies
- Minimizes consumption of water, disinfectants, and staff time
- Reduces staff exposure to fluid splashes, aerosols, or sharp injuries
- Often equipped with anti‑reflux valves and optional odor control
2.3 Challenges & Considerations
- Higher upfront cost compared to reusable canisters
- Increased plastic waste
- Dependence on uninterrupted supply chains during logistical disruptions
2.4 Safe Disposal
- Seal the liner immediately after use
- Place in red biohazard waste bags following regulatory guidelines
- If leakage occurs, use a secondary containment vessel

Section Three: Technical Comparison of Suction Systems
|
Performance Metric |
Reusable Canisters |
Disposable Suction Liner bag |
|
Biological Safety |
Depends on meticulous cleaning |
Safer – minimal exposure risk |
|
Staff Workload |
High (cleaning required) |
Very low (quick replacement) |
|
Initial Cost |
Lower |
Higher |
|
Operational Cost |
Higher (water, cleaning agents) |
Lower |
|
Training Requirements |
Intensive |
Simple |
|
Infection Control |
Varies, high risk if flawed |
Consistent, standardized |
|
Environmental Sustainability |
Less plastic waste |
More plastic—but potentially recyclable |
|
Wastewater Production |
Generates wastewater |
None |
Section Four: Practical Recommendations for Hospital Administrators
- In high‑risk and high‑volume areas (ICUs, operating rooms), prioritize the use of disposable liners.
- In resource-limited settings, implement reusable canisters with strict adherence to cleaning protocols.
- Offer ongoing training on proper cleaning techniques for both systems.
- Maintain standardized cleaning logs to monitor compliance.
- Evaluate eco-friendly, recyclable liner options to reduce plastic waste.
Final Conclusion
Fluid-collection devices are central to infection control in healthcare settings. While reusable canisters require rigorous protocols, time, and resources, disposable suction liners eliminate cleanup steps and dramatically reduce contact risk. Though liners entail higher initial costs, their long-term savings in labor, consumables, infection prevention, and operational efficiency make them a superior solution.
The optimal infection-control strategy combines both systems based on hospitalization risk, resources, and logistics.
References
- WHO. Decontamination and Reprocessing of Medical Devices for Health-care Facilities. Geneva: WHO, 2016.
- CDC. Guideline for Disinfection and Sterilization in Healthcare Facilities. Atlanta: CDC, 2008.
- AAMI. Comprehensive Guide to Medical Device Cleaning. 2021.
- Rutala WA, Weber DJ. Principles of Disinfection, Sterilization, and Antisepsis. 2019.
- Infection Control Today. Disposable Suction Liners and Infection Prevention, 2020.
- European Centre for Disease Prevention and Control. Infection Control Measures in Healthcare Settings, 2021.